Alan Frazer, Ph.D.
Professor/Research of Pharmacology
Personal Statement:
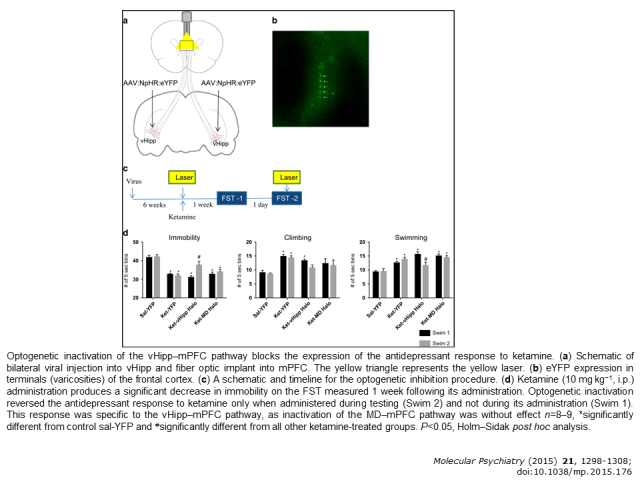
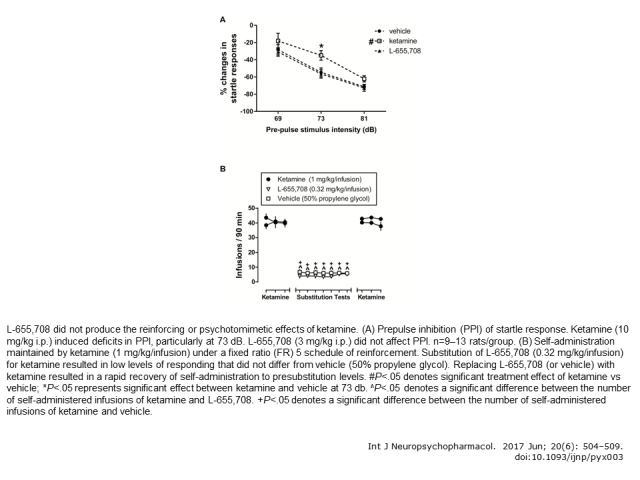
Although recently retired, my line of research is continuing to be carried out by collaborators in the Department with my former lab members now working directly with them and my still being involved- but at a distance. My primary research interest has been the mechanism of action of antidepressant drugs. Historically, the focus of my lab was to study how chronic treatment of rats with antidepressants affects the functioning of two monoamine systems, noradrenergic and serotonergic, that are important targets for their clinical effects. More recently, interest in treatment refractory depression led us to study two treatments for it, vagal nerve stimulation or ketamine. These studies focused on both the mechanisms of action of these treatments and the circuits in brain necessary for their beneficial effects. The latter studies have been carried out in collaboration with Dr. Daniel Lodge in this Department, using state-of-the-art techniques such as optogenetics and designer receptors exclusively activated by designer drugs (DREADDs). A Research Assistant Professor in the Department, Dr. Flavia Carreno (pictured in Lab Members), has been the lead investigator for these studies. This work led us to study effects of selective negative allosteric modulators of α5-GABAA receptors. Such drugs were found to have antidepressant-like effects similar to those of ketamine but not having ketamine’s adverse effect profile. Finally, in collaboration with another faculty member, Dr. David Morilak, both cognitive and emotional behaviors in animal models of depression or PTSD have been studied and the effect that antidepressant have on such behaviors. A graduate student, Aleeza Stephens (pictured in Lab Members), led this work which is continuing under the direction of Dr. Morilak.


Education
Ph.D., University of Pennsylvania
Research
| • antidepressants | • norepinephrine |
| • serotonin transporters | • vagal nerve stimulation |
Affiliations
Dr. Frazer is a member of numerous societies including the American Society of Pharmacology and Experimental Therapeutics, the American College of Neuropsychopharmacology (of which he has been the President), the International College of Neuropsychopharmacology (CINP), of which he is a Councillor, and the Editor-in-Chief of their official publication, International Journal of Neuropsychopharmacology and the Society of Neuroscience. He is on the Scientific Advisory Board of the Brain and Behavior Research Foundation, which awards NARSAD grants. He has been awarded a Merit Award from NIH and has been a Career Scientist of the Department of Veterans Affairs.

