April Risinger, Ph.D.
Associate Professor and Greehey Distinguished Chair in Targeted Molecular Therapeutics
Personal Statement:
The research in the Risinger Laboratory is in
the area of cancer pharmacology with a focus on microtubule targeted agents (MTAs) and other natural products for the treatment of triple-negative breast cancer (TNBC) and other solid tumors. There are four overarching goals of our research including the identification of biomarkers to improve the rational use of currently approved MTAs in cancer treatment, the development of novel MTAs that retain efficacy in drug-resistant disease, the discovery and development of other natural products that have efficacy against cancers that lack targeted treatment options, and identifying strategies to alleviate toxicities associated with MTAs and other chemotherapeutics to improve the quality of life of cancer patients. We collaborate closely with clinicians at UT Health as well as laboratories around the world that have complementary expertise in natural products chemistry, medicinal chemistry, and biotherapeutics to provide a multidisciplinary training environment and advance the development of new strategies to improve the treatment of cancer.
with a focus on microtubule targeted agents (MTAs) and other natural products for the treatment of triple-negative breast cancer (TNBC) and other solid tumors. There are four overarching goals of our research including the identification of biomarkers to improve the rational use of currently approved MTAs in cancer treatment, the development of novel MTAs that retain efficacy in drug-resistant disease, the discovery and development of other natural products that have efficacy against cancers that lack targeted treatment options, and identifying strategies to alleviate toxicities associated with MTAs and other chemotherapeutics to improve the quality of life of cancer patients. We collaborate closely with clinicians at UT Health as well as laboratories around the world that have complementary expertise in natural products chemistry, medicinal chemistry, and biotherapeutics to provide a multidisciplinary training environment and advance the development of new strategies to improve the treatment of cancer.
Drug Discovery and Development
Drug discovery and development projects in the laboratory include the identification and biological characterization of compounds that have selective activity against molecularly distinct TNBC subtypes and other solid tumors with the goal of identifying new targeted therapeutics for drug resistant disease. We collaborate closely with natural product chemists to identify and characterize novel natural compounds for their potential as anticancer agents as well as with synthetic and medicinal chemists to optimize their translation into the clinic. This includes our long-standing work on the plant-derived taccalonolide microtubule stabilizers. My laboratory found that the taccalonolides (taccas) bind covalently to a distinct site on tubulin from other MTAs with a high degree of specificity that allows them to retain efficacy in clinically relevant drug-resistant models both in vitro and in vivo. I am an inventor on two issued patents on this class of microtubule stabilizers and we have active projects in the laboratory focused on optimizing their pharmacokinetic properties and improving tumor targeting. Other active projects in this area include the repurposing of ingenane diterpenoids for the biomarker-guided treatment of TNBC subtypes that do not respond to currently approved chemotherapeutics.
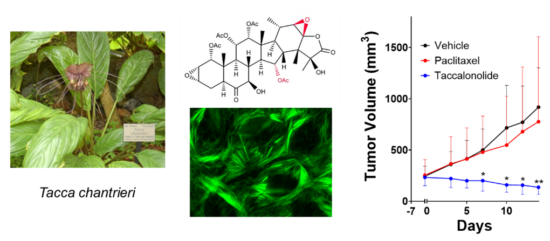
Improving the Targeted use of Approved Chemotherapeutics
In addition to identifying new therapeutics, my laboratory is also actively investigating the underappreciated mechanistic effects of MTAs currently approved for the treatment of cancer to guide their more rational and targeted use in the clinic. We have identified that the microtubule destabilizer eribulin is unique from the microtubule stabilizing taxanes in its ability to activate the cGAS-STING pathway through a mechanism involving mitochondrial DNA release. We further found that eribulin is highly synergistic with STING agonists, promotes the activation of antitumor NK cells, and that these immunological effects of eribulin are sufficient to promote antitumor immunity in vivo. We are also uncovering differences between the effects of clinically approved MTAs on cytoskeletal mediators implicated in oncogenesis, including the septins. These preclinical findings have led to active collaborations with clinicians at our Mays Cancer Center where we are conducting translational studies with the goal of developing a more rational, biomarker driven use of MTAs to improve patient response and mitigate toxicity.
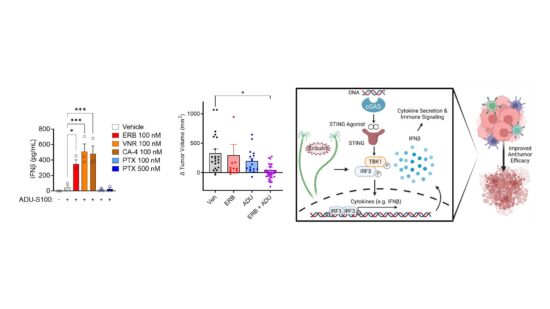
Improving treatment by alleviating toxic side effects
Some of the major hurdles to effective cancer treatment are the toxic side effects associated with MTAs and other chemotherapeutics. I am actively collaborating with neuroscientists and pain researchers to characterize and alleviate the neuropathic and cognitive deficits associated with cancer therapy. These collaborations have led to the development of models that allow monitoring of both the neurological and antitumor activities of chemotherapeutics in the same animal so that we can identify interventions that mitigate the deleterious side effects of these drugs without compromising their anticancer efficacy.
Education
BS Biochemistry, Texas A&M University, College Station, TX
PhD Cellular Biology, Massachusetts Institute of Technology, Cambridge, MA
Research
| • drug discovery | • cancer |
| • natural products | • microtubule targeted agents |
| • taccalonolides |
Awards & Accomplishments
Select Honors and Awards
2000 Best Undergraduate Thesis in Molecular Genetics, Texas A&M University
2012 Barbara Bowman Postdoctoral Award and Fellowship
2017 Voelcker Young Investigator Award
2022 Jack L. Beal Award for best paper by an earlier career investigator in the
Journal of Natural Products
Affiliations
Appointments
2008-present Member, Mays Cancer Center, UT Health San Antonio
2014-2018 Assistant Professor/Research Track, UT Health San Antonio
2018-2022 Assistant Professor/Tenure Track, UT Health San Antonio
2022-present Associate Professor with Tenure, UT Health San Antonio
Select Boards, Committees and Memberships
2017-2020 Scientific Advisory Board, Terrona LLC, San Antonio, TX
2019-present Physiology and Pharmacology Discipline Graduate Student Oversight Committee
2021-present Mays Cancer Center Shared Resource Committee
2022-present Center for Innovative Drug Discovery Steering Committee
2022 VPR Task Force for Women in Science
2022-present Physiology and Pharmacology Graduate Student Recruitment Committee
2022-present Standing Member, DMP Study Section, NIH
Lab Members

Jycole Bush
Mia Ramos
Past Lab Members
|
Publications
Selected Publications
Takahashi-Ruiz L, Morris JD, Crews P, Johnson TA, Risinger AL. In Vivo Evaluation of (-)-Zampanolide Demonstrates Potent and Persistent Antitumor Efficacy When Targeted to the Tumor Site. Molecules 27(13):4244. 2022.
Yee SS, Risinger AL. Efficacy of a covalent microtubule stabilizer in taxane-resistant ovarian cancer models. Molecules 26(13):4077. 2021
Fermaintt CS, Takahashi-Ruiz L, Liang H, Mooberry SL, Risinger AL. Eribulin activates the cGAS-STING pathway via the cytoplasmic accumulation of mtDNA. Molecular Pharmacology doi:10.1124/molpharm.121.000297. 2021
Risinger AL, Hastings S, Du L. Taccalonolide C-6 analogues, including paclitaxel hybrids, demonstrate improved microtubule polymerizing activities. The Journal of Natural Products 84(6):1799-1805. 2021. *Paper selected for the Jack L. Beal Award from the American Society of Pharmacognosy
Fermaintt CS, Peramuna T, Cai S, Takahashi-Ruiz L, Essif JN, Grant CV, O’Keefe BR, Mooberry SL, Cichewicz RH, Risinger AL. Yuanhuacine is a potent and selective inhibitor of the basal-like 2 subtype of triple negative breast cancer with immunogenic potential. Cancers 13(11):2834. 2021.
Matthew S, Chen QY, Ratnayake R, Fermaintt CS, Lucena-Agell D, Bonato F, Prota AE, Lim ST, Wang X, Díaz JF, Risinger AL, Paul VJ, Oliva MÁ, Luesch H. Gatorbulin-1, a distinct cyclodepsipeptide chemotype, targets a seventh tubulin pharmacological site. Proceedings of the National Academy of Sciences USA (PNAS) 118(9): e2021847118. 2021.
Du L, Yee SS, Ramachandran K, Risinger AL. Elucidating target specificity of the taccalonolide covalent microtubule stabilizers employing a combinatorial chemical approach. Nature Communications 11(1):654. 2020.
Clanton NA, Hastings SD, Foultz GB, Contreras JA, Yee SS, Arman HD, Risinger AL, Frantz DE. Synthesis and biological evaluations of electrophilic steroids inspired by the taccalonolides. ACS Medicinal Chemistry Letters 11(12):2534-2543. 2020.
Sharp AM, Lertphinyowong S, Yee SS, Paredes D, Gelfond J, Johnson-Pais TL, Leach RJ, Liss M, Risinger AL, Sullivan AC, Thompson IM, Morilak DA. Vortioxetine reverses medial prefrontal cortex-mediated cognitive deficits in male rats induced by castration as a model of androgen deprivation therapy for prostate cancer. Psychopharmacology 236(11):3183-3195. 2019.
LoCoco PM, Risinger AL, Smith HR, Chavera TS, Berg KA, Clarke WP. Pharmacological augmentation of nicotinamide phosphoribosyltransferase (NAMPT) protects against paclitaxel-induced peripheral neuropathy. eLife 10(6): E29626. 2017.
Du L, Risinger AL, Mitchell CA, You J, Stamps BW, Pan N, King JB, Bopassa JC, Judge SIV, Yang Z, Stevenson BS, Cichewicz RH. Unique amalgamation of primary and secondary structural elements transform peptaibols into potent bioactive cell-penetrating peptides. Proceedings of the National Academy of Sciences USA (PNAS) 114(43):E8957-E8966. 2017.


 Manasa Ravi
Manasa Ravi
 Samantha Yee, Ph.D.
Samantha Yee, Ph.D. Charles Fermaintt, Ph.D.
Charles Fermaintt, Ph.D. Leila Takahashi-Ruiz
Leila Takahashi-Ruiz Jacob Essif
Jacob Essif Nancy Wilkinson
Nancy Wilkinson