
Contact
- (210) 450-8157
- tumanov@uthscsa.edu
Department
Microbiology, Immunology & Molecular GeneticsTumanov, Alexei, M.D., Ph.D.
Associate Professor
Personal Statement:
Research in Dr. Alexei Tumanov`s lab investigates how the immune system regulates the delicate balance between protective immunity and immunopathology. The goal of Dr. Tumanov’s research program is to combine molecular data with in vivo models to understand the mechanisms underlying homeostatic and pathological conditions, thereby developing effective immunotherapies.
Dr. Tumanov graduated from the Russian State Medical University, Moscow, Russia. He did his PhD work in the laboratory of Dr. Sergei Nedospasov at the Engelhardt Institute of Molecular Biology, Moscow, in collaboration with several laboratories in Europe and the US, including NCI-Frederick (NIH), the Jackson Laboratory (Dr. Chervonsky`s lab), and the Institute of Experimental Immunology, Zurich (Dr. Zinkernagel`s lab). In 2004, Alexei joined the laboratory of Dr. Yang-Xin Fu at the University of Chicago, Department of Pathology, for postdoctoral training. In 2011, Alexei started his research lab at Trudeau Institute, NY. Dr. Tumanov was recruited to the Department of Microbiology, Immunology, and Molecular Genetics as an Associate Professor in Fall 2016.
Education
M.D., Russian State Medical University, Moscow
“Oncoimmunology” Educational Program in Immunology and Cancer Biology, Cancer Research Institute and Moscow State University (www.oncoimmunology.ru)
Ph.D., Molecular Biology, Engelhardt Institute of Molecular Biology, Russian Academy of Sciences, Moscow
Research
Our primary research focuses on understanding the biology of lymphotoxin (LT) and TNF cytokines. Over the last 20 years, we have developed various mouse models with targeted disruption of LT and TNF pathways, which serve as valuable tools for dissecting the underlying mechanisms in both homeostatic and pathological conditions. Our ongoing studies focus on the role of LT signaling in regulating pain responses, mucosal immunity, lymphoid organ homeostasis, and cancer immunotherapy.
1) Immune regulation of pain.
Chronic pain is a major health issue that has contributed to an epidemic of opioid misuse and overdose. Ongoing inflammation is linked to chronic pain; however, the cellular and molecular mechanisms behind this are not well understood. This project aims to investigate the immune mechanisms that regulate inflammatory pain, chemotherapy-induced peripheral neuropathic pain, and SARS-CoV-2-related pain, and to develop new immunotherapy strategies to reduce both acute and chronic pain. This research is being conducted in collaboration with Dr. Armen Akopian (Endodontics Department, UTHSCSA).

2) Regulation of effector CD8+ T cell elimination to manage immunopathology.
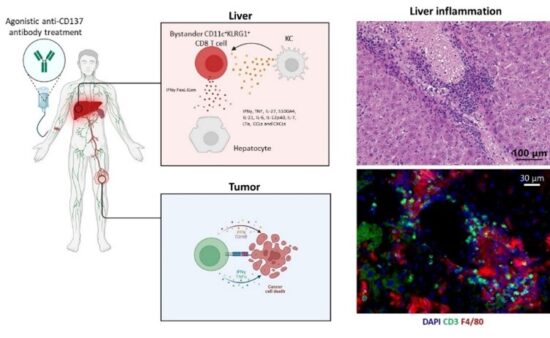
CD8+ T cell-driven pathologies present a significant challenge in cancer immunotherapy, as well as in the treatment of graft-versus-host disease, autoimmune disorders, and viral infections. Since CD8+ T cells are vital for protection, broad immunosuppression can weaken the host’s defenses, leading to reduced anti-tumor activity and increased risk of infections. The costimulatory molecule CD137 is an appealing target for immunotherapy in cancer and infectious diseases because it boosts cytotoxic CD8+ T cell responses. However, systemic activation of CD137 leads to a profound, antigen-independent expansion of effector CD8+ T cells, which results in hepatotoxicity. We study the mechanisms that control the removal of effector CD8+ T cells, aiming to develop strategies to ameliorate these T cell-driven pathologies.
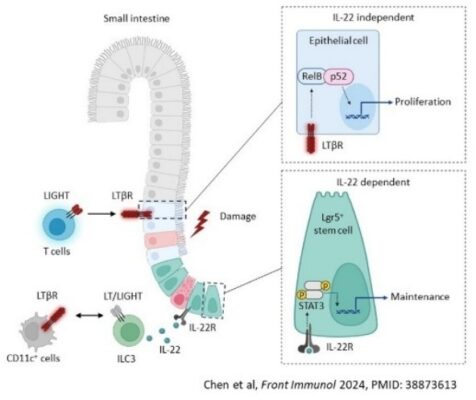
3) LTβR-dependent control of mucosal immune homeostasis in the gut and lung.
Besides lymphoid organs, LTβR signaling also plays a role in regulating mucosal immune responses in the gut and lung. Previously, we demonstrated that LTβR is essential for controlling ILC3 responses in defending against the mucosal bacterial pathogen Citrobacter rodentium (Immunity 2010, PMID: 20226692), and identified LT as a key regulator of ILC3 and IL-22 protective responses (Cell Host Microbe 2011, PMID: 21767811). Using mice with conditional inactivation of LTβR, developed in our lab, we showed that LTβR signaling in intestinal epithelial cells, dendritic cells, and neutrophils distinctly contributes to generating protective immune responses to mucosal pathogens, regulating metabolism, and repairing mucosal tissue after epithelial injury (Mucosal Immunol 2015, PMID: 25183367; Mucosal Immunol 2021, PMID: 33568785). Recently, we demonstrated the critical role of LIGHT-LTβR-RelB signaling in protecting against intestinal damage caused by chemotherapy (Front Immunol 2024, PMID: 38873613). This project aims to understand the LTβR-dependent mechanisms that control inflammation and mucosal repair, and to explore the potential of targeting this pathway in disease.
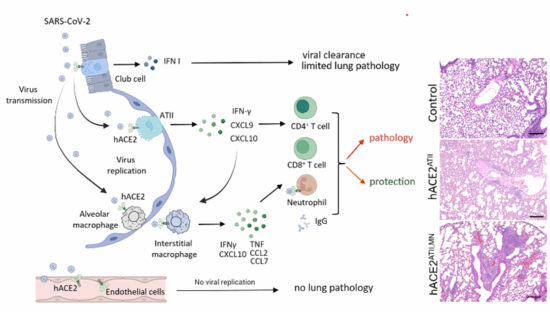
4) Regulation of immunopathology and protective immunity in SARS-CoV-2 infection.
We are interested in the role of distinct lung epithelial and immune cells in lung immunopathology and the generation of protective immune responses. To model SARS-CoV-2 infection in mice, we generated mice with cell-specific expression of hACE2, the receptor for SARS-CoV-2. Additionally, we are interested in mechanisms of extrapulmonary SARS-CoV-2 infection and pathogenesis of long COVID.
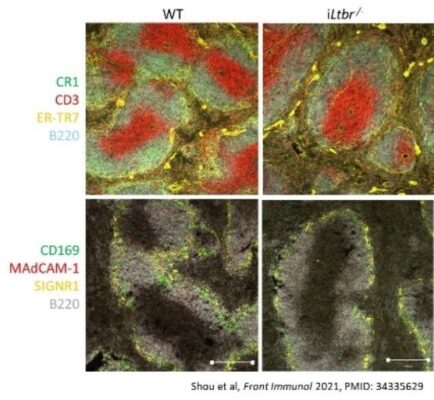
5) Lymphotoxin signaling in the homeostasis of lymphoid organs.
Lymphotoxin beta receptor (LTβR) is a crucial regulator of lymphoid organ development and maintenance. We created mice with conditional inactivation of LTβ and LTβR, allowing us to determine the role of specific LTβ- and LTβR-producing cells in protective immunity and immunopathology. Recent multi-national research identified and characterized patients with biallelic loss-of-function mutations in LTβR, which resulted in a complete absence of lymph nodes and impaired B cell differentiation, further supporting prior 30-year research using LTβ- and LTβR-deficient mice (Sci Immunol 2024, PMID: 39576873). The ability of LTβR signaling to enhance immune responses and facilitate the formation of tertiary lymphoid organs can be utilized in cancer immunotherapy and autoimmune diseases. The goal of this project is to develop an LTβR-targeted pathway in cancer immunotherapy and autoimmune diseases.
Awards & Accomplishments
- 1999 Research Training Fellowship of the International Agency for Research on Cancer
- 2001 Outstanding Scholar Award. International Cytokine Society
- 2002 International Union Against Cancer (IUCC), Cancer Technology Transfer Fellowship
- 2004 Grant from the Russian Foundation for Basic Research
- 2007 AAI and Keystone Conference Junior Faculty Travel Awards
- 2007 Scientist Development Grant, American Heart Association
- 2008 Pilot and Feasibility Award, University of Chicago Digestive Diseases Research Center
- 2010 Career Development Award, Crohn’s and Colitis Foundation of America
- 2012 CCFA Shanthi Sitaraman Memorial Young IBD Investigator Award
- 2013 Junior Faculty Travel Award, 14th International TNF Conference
- 2014 Biomedical Research Grant, American Lung Association
- 2014 Senior Research Award, Cohn’s and Colitis Foundation of America
- 2017 The Max and Minnie Tomerlin Voelcker Fund Investigator Award
- 2018 Peter Bradley Carlson Trust Award
- 2019 The William and Ella Owens Medical Research Foundation Award
- 2022 Cancer Prevention and Research Institute of Texas Investigator Award
Lab Members
Austin Todd
MD/PhD Student


Sergey Shein, PhD
Instructor/Research

Ekaterina Koroleva, PhD
Assistant Professor/Research

Anna Korchagina, PhD
Instructor/Research





Alumni
Brianna Faz, Research Assistant, 2023-2025 – Currently: MD Student, UT Southwestern
Vasily Kudinov, PhD, Visiting Scientist, 2024 – Currently: Biotech Company
Ishana Khetarpal, Research Assistant, 2024 – Currently: MD Student, UTMB
Amanda Muñoz, Postdoctoral Fellow, 2018-2022 – Currently: Assistant Professor, Texas A&M International University
Qiangxing Chen, Visiting Student, 2022-2024 – Currently: MD Resident, First Affiliated Hospital of Guangxi Medical University, China
Justin Nguyen, MS Student, 2022-2024 – PhD Student, UTMB
Yajun Shou, Visiting Student, 2020-2022 – MD, Department of Gastroenterology, The Second Xiangya Hospital, Central South University, Changsha, China
Jing Xi, Visiting Student, 2020-2022 – Currently: School of Nursing, Jinan University, Guangzhou, China
Kizil Yusoof, PhD Student, 2022 – Currently: PhD Public Health, University of Arizona
Jensine Vallecer, MS Student
Wayne Muraoka, PhD, Postdoctoral Fellow, 2015-2017 – Currently: Director of Research, Certified Group, San Antonio, TX
Ekaterina Gubernatorova, MS Student, PhD Student, 2014-2022 – Currently: Senior Staff Scientist, Engelhardt Institute of Molecular Biology, Moscow, Russia
Cody Spencer, Postdoctoral Fellow, 2013-2015 – Currently- Senior Scientist, BioXCell.
Elise Macho-Fernandez, PhD, Postdoctoral Fellow – Currently: Medpace, Lyon France
Luke Neill, B.S. Research Assistant, 2012 – Currently: M.D., Clinical Assistant Professor, Northwestern Medicine, Chicago, IL
Publications
- Korchagina AA, Shein SA, Muraoka WT, Nguyen J, Chen Q, Tumanova AA, Todd AW, Rivera CE, Tamayo R, Casali P, Koroleva E, Tumanov AV. Tofacitinib ameliorates Campylobacter-induced intestinal pathology by suppressing IFNγ producing ILCs and T cells. Mucosal Immunol. 2025 Jul 3: PMID: 40614970.
- Kuppusamy M, Ottolini M, Chen Y-L, Daneva Z, Li J , Heng-Mae Cheung C, Rios N, Radi R, Garcia G, Nwafor D, Park MS, Tumanov AV, Sonkusare SK. Paracrine smooth muscle-to-endothelial signaling via TNF elevates blood pressure in obesity. Circ Res, 2025 Aug 1:10.1161/CIRCRESAHA.124.326069. PMID: 40747548.
- Erlich EC, Czepielewski RS, Field RL, Dunning TJ, Saleh LS, Hoofnagle MH, Tumanov AV, Guilak F, Brestoff JR, Randolph GJ. Distinct roles for LTa3 and LTa1b2 produced by B cells contribute to their multi-faceted impact on ileitis. Nat Immunol 2025, Sep 8. PMID: 40921841.
- Boylan BT, Hwang M, Brozost E, Oh H, Tumanov AV, Louveau A, Bergmann CC. B cells are not drivers of stromal cell activation during acute CNS infection. J Neuroinflammation 2025 Jun 27;22(1):165. PMID: 4057970, PMC12203728.
- Amisaki M, Zebboudj A, Yano H, Zhang SL, Payne G, Chandra AK, Yu R, Guasp P, Sethna ZM, Ohmoto A, Rojas LA, Cheng C, Waters T, Solovyov A, Martis S, Doane AS, Reiche C, Bruno EM, Milighetti M, Soares K, Odgerel Z, Moral JA, Zhao JN, Gönen M, Gardner R, Tumanov AV, Khan AG, Vergnolle O, Nyakatura EK, Lorenz IC, Baca M, Patterson E, Greenbaum B, Artis D, Merghoub T, Balachandran VP. IL-33-activated ILC2s induce tertiary lymphoid structures in pancreatic cancer. Nature 2025 Feb;638(8052):1076-1084. PMID: 39814891.
- Chen Q, Muñoz AR, Korchagina AA, Shou Y, Vallecer J, Todd AW, Shein SA, Tumanov AV*, Koroleva E*. LTβR-RelB signaling in intestinal epithelial cells protects from chemotherapy-induced mucosal damage. Front Immunol. 2024 May 30;15:1388496.*- Contributed equally. PMID: 38873613.
- Ransmayr B., Köstel SB, Thian M, Svaton M, van de Wetering C, Hafemeister C, Segarra A, M.Sc., Block J, Frohne A, Altunbas MY, Eltan SB, Kıykım A, Aydiner O, Kesim S, Inanir S, Karakoc-Aydiner E, Ozen A, Aba U, Çomak A, Tuğcu GD, Huber B, Farlik M, Simonitsch-Klupp I, Halbritter F, Tumanov AV, Kraakman MJ, Metin A, Castanon I, Erman B, Baris S, Kaan Boztug K. Lymph node aplasia and impaired B-cell maturation in human LTβR deficiency. Sci Immunology, 2024 Nov 22;9(101):eadq8796. PMID: 39576873.
- Naratadam GT, Mecklenburg J , Shein SA , Yi Zou, Zhao Lai, Tumanov AV, Price TJ, Akopian AN. Sci Rep. 2024 Jul 30;14(1):17543. Degenerative and regenerative peripheral processes are associated with persistent painful chemotherapy-induced neuropathies in males and females. PMID: 39080341.
- Korchagina AA, Koroleva E, Tumanov AV. Innate lymphoid cell plasticity in mucosal infections. Microorganisms. 2023 Feb 12;11(2):461. PMID: 36838426.
- Korchagina AA, Shein SA, Koroleva E, Tumanov AV. Transcriptional control of ILC identity. Front Immunol. 2023 Mar 9;14:1146077. PMID: 36969171.
- Lindquist KA, Shein SA, Hovhannisyan AH, Mecklenburg J, Zou Y, Lai Z, Tumanov AV, Akopian AN. Associations of tissue damage induced inflammatory plasticity in masseter muscle with the resolution of chronic myalgia. Sci Rep. 2023 Dec 12;13(1):22057. doi: 10.1038/s41598-023-49280-1. PMID: 38086903.
- Mecklenburg J, Shein SA, Hovhannisyan AH, Zou Y, Lai Z, Ruparel S, Tumanov AV, Akopian AN. Transcriptional Profiles of Non-neuronal and Immune Cells in Mouse Trigeminal Ganglia. Front Pain Res 2023, doi: 10.3389/fpain.2023.1274811. PMID: 38028432.
- Lim VY, Feng X, Miao R, Zehentmeier S, Ewing-Crystal N, Lee M, Tumanov AV, Oh JE, Iwasaki A, Wang A, Choi J, Pereira JP. Mature B cells and mesenchymal stem cells control emergency myelopoiesis. Life Sci Alliance. 2023, Jan 30;6(4):e202301924. PMID: 36717247.
- Miki H, Kiosses WB, Manresa MC, Gupta RK, Sethi GS, Herro R, Da Silva Antunes R, Dutta P, Miller M, Fung K, Chawla A, Dobaczewska K, Ay Ferhat, Broide DH, Tumanov AV, Croft M. Lymphotoxin beta receptor signaling directly controls airway smooth muscle deregulation and asthmatic lung dysfunction. J Allergy Clin Immunol. 2023, Apr;151(4):976-990.e5. PMID: 36473503.
- Zehentmeier S, Lim VY, Ma Y, Fossati J, Ito T, Jiang Y, Tumanov AV, Lee HJ, Dillinger L, Kim J, Csomos K, Walter JE, Choi J, Pereira JP. Dysregulated stem cell niches and altered lymphocyte recirculation cause B and T cell lymphopenia in WHIM syndrome. Sci Immunol. 2022, Sep 23;7(75):eabo3170. PMID: 36149943.
- Mecklenburg J, Wangzhou A, Hovhannisyan AH, Barba-Escobedo P, Shein SA, Zou Y, Weldon K, Lai Z, Goffin V, Dussor G, Tumanov AV, Price TJ, Akopian AN. Sex-dependent pain trajectories induced by prolactin require an inflammatory response for pain resolution. Brain Behav Immun. 2022 Jan 19;101:246-263. PMID: 35065194.
- Korchagina AA, Koroleva E, Tumanov AV. Innate lymphoid cells in response to intracellular pathogens: protection versus immunopathology. Front Cell Infect Microbiol. 2021 Dec 6;11:775554. PMID: 34938670. PMCID: PMC8685334.
- Shou Y, Koroleva E, Spencer CM, Shein SA, Korchagina AA, Yusoof KA, Parthasarathy R, Leadbetter EA, Akopian AN, Muñoz AR, Tumanov AV. Redefining the role of lymphotoxin beta receptor in the maintenance of lymphoid organs and immune cell homeostasis in adulthood. Front Immunol 2021; 12:712632. PMID: 34335629, PMCID: PMC8320848.
- Muraoka WT, Korchagina AA, Xia Q, Shein SA, Jing X, Lai Z, Weldon KS, Wang LJ, Chen Y, Kummer LW, Mohrs M, Vivier E, Koroleva EP, Tumanov AV. Campylobacter infection promotes IFNγ-dependent intestinal pathology via ILC3 to ILC1 conversion. Mucosal Immunol 2021;14(3):703-716. PMID 33214656, PMCID: PMC8084871.
- Vanderkerken M, Baptista AP, Giovanni M, Fukuyama S, Browaeys R, Scott CL, Norris PS, Eberl G, Di Santo JP, Vivier E, Saeys Y, Hammad H, Cyster JG, Ware CF, Tumanov AV*, De Trez C*, Lambrecht BN*. *- Contributed equally. ILC3s control splenic cDC homeostasis via lymphotoxin signaling. J Exp Med 2021;218(5): e20190835. PMID: 33724364.
- Riffelmacher T, Giles DA, Zahner S, Dicker M, Andreyev AY, McArdle S, Perez-Jeldres T, van der Gracht E, Murray MP, Hartmann N, Tumanov AV, Kronenberg M. Metabolic activation and colitis pathogenesis is prevented by lymphotoxin β receptor expression in neutrophils. Mucosal Immunol 2021;14(3):679-690. PMID 33214656.
- Jing X, Korchagina AA, Shein SA, Muraoka WT, Koroleva E, Tumanov AV. IL-23 contributes to Campylobacter jejuni-induced intestinal pathology via promoting IL-17 and IFNγ responses by innate lymphoid cells. Front Immunol 2021;11: 579615. PMID: 33488580.
- Mecklenburg J, Zou Y, Wangzhou A, Garcia D, Lai Z, Tumanov AV, Dussor G, Price TJ, Akopian AN. Transcriptomic sex differences in sensory neuronal populations of mice. Sci Rep 2020;10(1):15278. PMID: 32943709, PMCID: PMC7499251.
- James K.D., Cosway E.J., Lucas B., White A.J., Parnell S.M., Carvalho-Gaspar M., Tumanov A.V., Anderson G., Jenkinson W.E. Endothelial cells act as gatekeepers for LTbR-dependent thymocyte emigration. J Exp Med, Nov 13. doi: 10.1084/jem.20181345, 2018. PMID: 30425120.
- Giles D.A., Zahner S., Krause P., van der Gracht E., Riffelmacher T., Morris V., Tumanov A.V., Kronenberg M. The tumor necrosis factor superfamily members TNFSF14 (LIGHT), lymphotoxin β and lymphotoxin β receptor interact to regulate intestinal inflammation. Front Immunol, doi: 10.3389/fimmu.2018.02585, 2018. PMID: 30524422.
- Gubernatorova E.O., Gorshkova E.A. Namakanova O.A., Zvartsev R.V., Hidalgo K, Drutskaya M.S., Tumanov A.V., Nedospasov S.A. Non-redundant functions of IL-6 produced by macrophages and dendritic cells in allergic airway inflammation. Front Immunol. Nov 26;9:2718, 2018. PMID: 30534125.
- Koroleva E.P., Fu Y.X., Tumanov A.V. Lymphotoxin in physiology of lymphoid tissues – Implication for antiviral defense. Cytokine. Jan;101:39-47. pii: S1043-4666(16)30472-0, 2018. PMID: 27623349.
- Schaeuble K, Britschgi M.R., Scarpellino L., Favre S., Xu Y., Koroleva E., Lissandrin T.K.A., Link A., Matloubian M., Ware C.F., Nedospasov S.A., Tumanov A.V., Cyster J.G., Luther S.A. Perivascular fibroblasts of the developing spleen act as LTα1β2-dependent precursors of both T and B zone organizer cells. Cell Rep. 2017 Nov 28, 21(9):2500-2514, 2017. PMID: 29186687.
- Cosway, E.J., Lucas B., James K.D., Parnell S.M., Carvalho-Gaspar M., White A.J., Tumanov A.V., Jenkinson W.E., Anderson G. Redefining thymus medulla specialization for central tolerance. J Exp Med. Nov 6, 214(11):3183-3195, 2017. PMID: 28830910.
- Bakshi S.F., Guz N., Zakharchenko A., Deng H., Tumanov A.V., Woodworth C.D., Minko S., Kolpashchikov D.M., Katz E. Magnetic field-activated sensing of mRNA in living cells. J Am Chem Soc. Sep 6, 139(35):12117-12120, 2017. PMID: 28817270.
- Gubernatorova E.O., Liu X, Othman A., Muraoka W.T, Koroleva E.P., Andreescu S., Tumanov A.V. Europium-doped cerium oxide nanoparticles limit reactive oxygen species formation and ameliorate intestinal ischemia-reperfusion injury. Adv Healthc Mater, May 8. doi: 10.1002/adhm.201700176, 2017. PMID: 28481012.
- Gubernatorova E.O., Koroleva E.P., Halperin S., Perez-Chanona E., Jobin C., Tumanov A.V. Murine model of intestinal ischemia-reperfusion injury. J Vis Exp, May 11;(111). doi: 10.3791/53881, 2016. PMID: 27213580.
- Macho-Fernandez E., Koroleva E.P., Spencer C.M., Tighe M., Torrado E., Cooper A.M, Fu Y-X, Tumanov A.V. Lymphotoxin beta receptor signaling limits mucosal damage through driving IL-23 production by epithelial cells. Mucosal Immunol, Mar, 8(2):403-13, 2015. PMID: 25183367.
- Koroleva E.P., Halperin S., Gubernatorova E.O., Spencer C.M, Tumanov A.V. Citrobacter rodentium- induced colitis: a robust model to study mucosal immune responses in the gut. J Immunol Methods. 421:61-72, 2015. PMID: 25702536.
- Wolf M.J., Adili A., Piotrowitz K., Abdullah Z., Boege Y., Stemmer K., Ringelhan M., Simonavicius N., Egger M., Wohlleber D., Lorentzen A., Einer C., Schulz S., Clavel T., Protzer U., Thiele C., Zischka H., Moch H., Tschöp M., Tumanov A.V, Haller D., Unger K., Karin M., Kopf M., Knolle P., Weber A., Heikenwalder M. Metabolic activation of intrahepatic CD8(+) T Cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell, Oct 13;26(4):549-64, 2014. PMID: 25314080.
- Kruglov A.A., Grivennikov S.I., Kuprash D.V., Winsauer C., Prepens S., Seleznik G. M., Heikenwalder M., Eberl G., Littman D.R., Tumanov A.V., Nedospasov S.A. Non-redundant function of soluble LTα3 produced by innate lymphoid cells in intestinal homeostasis. Science, 342(6163):1243-6, 2013. PMID: 24311691.
- Upadhyay V., Poroyko V., Kim T.J., Devkota S., Fu S., Liu D., Tumanov A.V., Koroleva E.P., Deng L., Nagler C., E. B. Chang, H. Tang, Y. X. Fu. Lymphotoxin regulates commensal responses to enable diet-induced obesity. Nat Immunol., 13(10): 947-53, 2012. PMID: 22922363.
- Moseman E. A., Iannacone M., Bosurgi L., Tonti E., Chevrier N., Tumanov A., Fu Y-X, N. Hacohen, von Andrian, U.H. 2012. B cell maintenance of subcapsular sinus macrophages protects against a fatal viral infection independent of adaptive Immunity. Immunity, 36(3):415-26, 2012. PMID: 22386268.
Tumanov AV, Koroleva EP, Guo X, Wang Y, Kruglov A, Nedospasov S, Fu YX. Lymphotoxin controls the IL-22 protection pathway in gut innate lymphoid cells during mucosal pathogen challenge. Cell Host Microbe, 10(1):44-53, 2011. PMID: 21767811. - Wang Y., Koroleva E.P., Kruglov A.A., Kuprash D.V., Nedospasov S.A., Fu Y-X, Tumanov A.V. Lymphotoxin beta receptor signaling in intestinal epithelial cells orchestrates innate immune responses against mucosal bacterial infection. Immunity, 32(3): 403-13, 2010. PMID: 20226692.
Chen L., Park S-M., Tumanov A.V., Hau A., Sawada K., Feig C., Turner J.R., Fu Y-X., Romero I., Lengyel E., Peter M.E. CD95/FAS promotes tumor growth. Nature, 465(7297):492-6, 2010. PMID: 20505730. - Tumanov A. V., Grivennikov S.I., Kruglov A.A., Shebzukhov Y.V., Koroleva EP, Piao Y., Cui X-Y., Littman D.R., Kuprash D.V. Nedospasov S.A. Cellular source and molecular form of TNF specify its distinct functions in organization of secondary lymphoid organs. Blood, 116 (18):3456-64, 2010. PMID: 20634375.
Tumanov A.V., Koroleva E.P., Christiansen P.A., Khan M.A., Ruddy M.J., Burnette B., Papa S., Franzoso G., Nedospasov S., Fu Y-X., Anders R.A. T cell derived lymphotoxin regulates liver regeneration. Gastroenterology, 136(2):694-704, 2009. PMID: 18952083. - Tumanov A.V., Christiansen P.A., Fu Y-X. The role of lymphotoxin receptor signaling in diseases. Current Mol. Medicine, 7: 567-578, 2007. PMID: 17896993.
- Lo J.C.*, Wang Y.*, Tumanov A.V.*, Bamji M., Yao Z., Reardon C.A., Getz G.S., Fu Y-X. Lymphotoxin beta receptor-dependent control of lipid homeostasis. Science, 316(5822):285-8, 2007. * Contribute equally. PMID: 17431181.
- Junt, T., Tumanov, A.V., Harris, N., Heikenwalder, M., Zeller N.,, Kuprash D.K., Aguzzi A., Ludewig, B., Nedospasov, S.A., Zinkernagel, R.M. Expression of lymphotoxin beta governs immunity at two distinct levels. Eur J Immunol. 36(8):2061-75, 2006. PMID: 16841297.
- Grivennikov, S.I. *, Tumanov, A.V.*, Liepinsh, D. J., Kruglov, A.A., Marakusha, B.I., Shakhov, A.N., Murakami, T., Drutskaya, L.N., Förster, I., Clausen, B.E., Tessarollo, L., Ryffel, Bernhard, Kuprash, D.V., Nedospasov, S.A.: Distinct and non-redundant in vivo functions of TNF produced by T cells and macrophages/neutrophils: protective and deleterious effects. Immunity, 22 (1): 93-104, 2005. *Contribute equally. PMID: 15664162.
- Tumanov, A.V, Kuprash, D.V, Mach, J.A, Nedospasov, S.A, Chervonsky, A.V. Lymphotoxin and TNF produced by B cells are dispensable for maintenance of the follicle-associated epithelium but are required for development of lymphoid follicles in the Peyer’s patches. J Immunol. 173: 86-91, 2004. PMID: 15210762.
- Tumanov, A. V., Kuprash, D. V., and Nedospasov, S. A.: The role of lymphotoxin in development and maintenance of secondary lymphoid tissues. Cytok Growth Factor Rev. 14: 275-288, 2003. PMID: 12787565.
- Tumanov, A. V., Grivennikov, S. I., Shakhov, A. N., Rybtsov, S. A., Koroleva, E. P., Takeda, J., Nedospasov, S. A., and Kuprash, D. V.: Dissecting the role of lymphotoxin in lymphoid organs by conditional targeting. Immunol Rev. 195: 106-116, 2003. PMID: 12969314.
- Tumanov, A. V., Kuprash, D. V., Grivennikov, S. I., Lagarkova, M. A., Abe, K., Shakhov, A. N., Drutskaya, L. N., Stewart, C. L., Chervonsky, A. V., and Nedospasov, S. A.: Distinct role of surface lymphotoxin expressed by B cells in the organization of secondary lymphoid tissues. Immunity. 17: 239-250, 2002. PMID: 12354378.
- Kuprash, D. V. *, Alimzhanov, M. B. *, Tumanov, A. V. *, Shakhov, A. N., Grivennikov, S. I., Marino, M. W., Turetskaya, R. L., Anderson, A. O., Rajewsky, K., Pfeffer, K., and Nedospasov, S. A.: Redundancy in TNF and LT signaling in vivo: mice with inactivation of the entire TNF/LT locus versus single knockout mice. Mol. Cell. Biol. 22: 8626-8634, 2002. *Contribute equally. PMID: 12446781.
- Kuprash, D. V. *, Alimzhanov, M. B. *, Tumanov, A. V. *, Rajewsky, K., Anderson, A. O., Pfeffer, K., and Nedospasov, S. A.: Establishment of spleen microarchitecture, but not organogenesis of lymph nodes requires cooperation of tumor necrosis factor and lymphphotoxin beta. J. Immunol. 163: 6575-6580, 1999. *Contribute equally. PMID: 10586051.
- Tumanov, A.V., Nedospasov, S.A., Turetskaya, R.L. Chromatin organization in the tumor necrosis factor/lymphotoxin gene locus: correlation with tissue-specific expression. Molecular Biology (in Russian), 32(1): 102-106, 1998. PMID: 9566256.
