Contact
- (210) 567-3978
- griffithA3@uthscsa.edu
Department
Microbiology, Immunology & Molecular GeneticsGriffith, Ann V., Ph.D.
Associate Professor
Personal Statement:
Ann’s laboratory website can be found here.
Research in Dr. Ann Griffith’s laboratory focuses on the lymphopoietic stromal microenvironment in the thymus. T lymphocytes are critical mediators of immunity generated in the thymus through mutually inductive “cross-talk” with thymic stromal cells. Age-induced alterations in stromal cells cause substantial thymic atrophy and dysfunction, resulting in diminished T cell production and concomitant immunodeficiencies, including decreased responsiveness to infection and vaccination. Preserving thymus function therefore holds significant potential to extend the healthspan. However, mechanisms driving age-induced stromal dysfunction have been difficult to resolve because thymic stromal cells are rare and difficult to isolate. Her lab applies a novel computational deconvolution approach to spatially map stromal gene expression using microdissected whole tissue, eliminating the need for stromal cell isolation. Long-term goals of the lab include revealing novel lymphopoietic stromal functions in the young, steady state thymus, and boosting thymus function during aging.
Education
Ph.D., University of Texas Health Science Center at Houston-M.D. Anderson Cancer Center, 2006
Research
Ann’s laboratory website can be found here.
Dr. Griffith’s research interests lie in the identification of lymphopoietic signals provided by the stromal microenvironment in the thymus, the biology of thymic stromal cells, and the mechanisms and consequences (including diminished vaccine response and increased susceptibility to infection) of age-induced thymic atrophy. The impact of age-induced atrophy on T cell production, which is pronounced by young adulthood, is not limited to the elderly, but is also apparent in young adults when lymphopenia is actively induced, such as in patients receiving myeloablative therapy and bone marrow stem cell transplants. The primary targets of age-induced atrophy are a relatively rare stromal population. Unfortunately this population is difficult to isolate for a number of reasons, and the mechanisms governing thymic atrophy have remained relatively obscure. Over the last several years they have devised and implemented a novel computational deconvolution approach to globally and spatially map gene expression in thymic stromal cells, revealing exciting new aspects of thymus biology. Ongoing projects in the lab aim to identify the causes and consequences of age-associated thymic stromal dysfunction. Their ultimate goal is to develop novel approaches to extend the health span during aging.
Awards & Accomplishments
R21 AI166843 Griffith/Zhang/Xiang (MPI) 06/01/22-05/31/24 NIH
THE IMPACT OF AGING AND THYMUS REGENERATION ON TISSUE-RESIDENT CD8 T CELL RESPONSES TO VIRAL INFECTION AND VACCINATION
In this study we will test the hypothesis that thymus regeneration by growth hormone administration promotes T cell responses to vaccination and viral infection by improving tissue-resident T cell function in aged murine lung. Role: Contact MPI
R56 AI153626 Griffith (PI) 07/01/21-06/30/22 NIH. (NCE to 6/30/23)
CAUSES AND CONSEQUENCES OF DECLINING B CELL-MEDIATED CENTRAL T CELL TOLERANCE THROUGHOUT THE LIFESPAN
In this study we will test the hypothesis that declining thymic B cell expression of self-antigens impairs T cell tolerance and promotes autoimmunity. Role: PI
R21 AI154109 Griffith (PI) 02/15/21-01/31/23 NIH
THE ROLE OF PARACRINE MTOR SIGNALING IN REGULATING THYMUS SIZE AND FUNCTION
In this study we will test the hypothesis that paracrine mTOR signaling maintains thymus size using tissue-specific transgenic mice overexpressing mTOR activating ligands in medullary thymic epithelial cells (mTEC). Role: PI
| Year | Award |
|---|---|
| 2022 | Fall STAR Educator https://lsom.uthscsa.edu/aes/2023-star-educators-recipients/2023-star-educator-recipient-ann-griffith-phd-mimg/ |
| 2021 | Graduate School of Biomedical Science Mentor of the Year |
| 2018 | Howard Hughes Medical Institute Gilliam Fellowship Mentor https://lsom.uthscsa.edu/mimg/2018/08/15/sergio-cepeda-and-dr-ann-griffith-awarded-gilliam-fellowships-for-advanced-study |
| 2018 | Editorial Board: Aging Cell |
| 2017 | Max and Minnie Tomerlin Voelcker Fund Young Investigator Award |
| 2016 | Faculty of the Year, UT Health San Antonio Department of Microbiology, Immunology & Molecular Genetics |
| 2015 | American Association of Immunologists Early Career Travel Award |
| 2015 | Greehey President’s Fund for Faculty Excellence Award |
| 2014 | American Association of Immunologists Travel for Techniques Award |
Affiliations
American Association of Immunologists
Lab Members
| Picture | Name |
|---|---|
 | Yangming Xiao Ph.D. M.D Lab Manager XiaoY@livemail.uthscsa.edu |
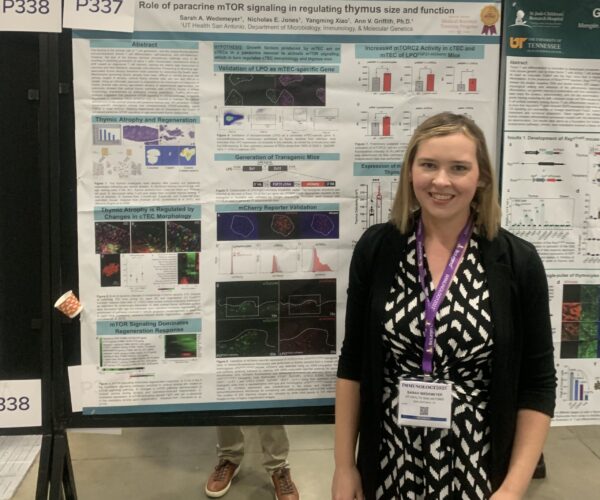
 | Sarah Wedemeyer M.D./Ph.D. Student wedemeyers@livemail.uthscsa.edu |
 | Kahealani Archuleta Research Assistant archuletak@livemail.uthscsa.edu |
 | Jaid Perez PhD Student perezj38@livemail.uthscsa.edu |
 | Brittan Burns PhD Student burnsb1@livemail.uthscsa.edu |
 | Tariq Elhashim PhD Student elhashim@livemail.uthscsa.edu |
Griffith Lab Alumni
- Nicholas Jones (M.S.)
- Carolina Cantu (M.S.)
- Stephanie Orozco (M.S.)
- Abdulaziz Almutairi (M.S.)
- Martin Sandoval (M.S.)
- Stephanny Lizarraga (M.S.)
- Chioma Udeaja (M.S.)
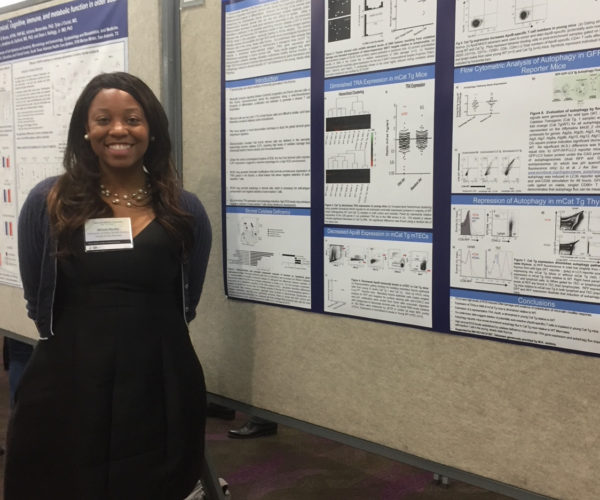
- Kymberly Wimberly (M.S.)
- Allison Hester (Ph.D.)
- Sergio Cepeda (Ph.D.)
- Manpreet Semwal (Ph.D.)
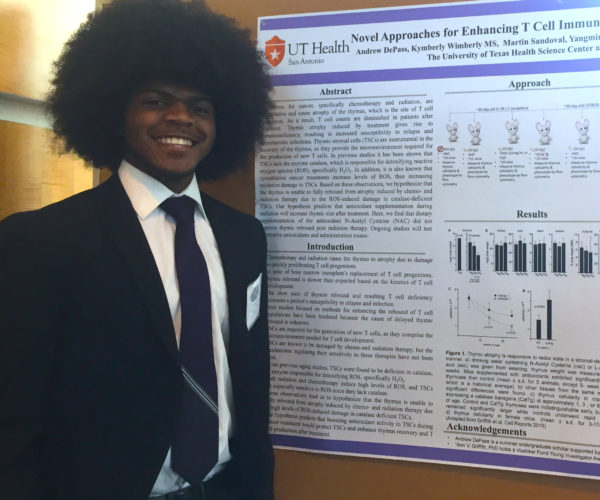
- Aaron Garza (M.S.)
News
- Congratulations to Aaron for earning the American Legion Post 336 Scholarship for Veterans 2023!
- Congratulations to Kahealani for earning the UTHSCSA Bennie W. Schreck Scholarship 2023!
- Congratulations to Sarah for winning 2nd place at the South Texas MSTP Spring Retreat Poster Session!
- Congratulations to Sarah for earning an AAI Travel Award and podium presentation spot for Immunology 2023!
- 06/2024 Congratulations to Aaron and Kahealani for successfully defending their M.S. Theses!
- Congratulations to Sarah for her first-place win at the annual Conklyn Symposium!
- 04/2024 Sarah continued her winning streak by tying for first place at the MSTP 3-minute thesis competition!
- Congratulations to Sarah for being selected as the M.D./Ph.D. student speaker at the Long School of Medicine Research Symposium 2024!
- 02/2024 Sarah was selected to give a trainee talk in the Major Symposium “New Insights into Thymic Selection, Tolerance, and Regeneration” at AAi 2024. Way to go Sarah!
- 10/2023 Congrats to the Griffith Lab, 2023 MIMG Pumpkin Decorating Contest 2nd Place Winners!
Publications
Wedemeyer, S.A., Jones, N.E., Raza, I.G., Green, F. M., Xiao, Y., Semwal, M.K., Garza, A.K., Archuleta, K.S., Wimberly, K.L., Venables, T., Hollander, G.A., Griffith, A.V., 2025 Paracrine FGF21 dynamically modulates mTOR signaling to regulate thymus function across the lifespan. Nature Aging Feb 19;5(4):588-606. PMID: 39972173. https://pmc.ncbi.nlm.nih.gov/articles/PMC12003089/
Semwal, M.K., Hester, A,K., Xiao, Y., Udeaja, C., Cepeda, S., Verschelde, J.S., Jones, N., Wedemeryer, S.A., Emtage, S., Wimberly, K., 0Griffith, A.V. 2022 Redox status regulates autophagy in thymic stromal cells and promotes T cell tolerance. PNAS 119 (40) e2204296119. doi.org/10.1073/pnas.2204296119 PMCID: PMC9549397.https://www.pnas.org/doi/full/10.1073/pnas.2204296119
Hester AK, Semwal MK, Cepeda S, Xiao Y, Rueda M, Wimberly K, Venables T, Dileepan T, Kraig E, Griffith AV. Redox regulation of age-associated defects in generation and maintenance of T cell self-tolerance and immunity to foreign antigens. Cell Rep. 2022 Feb 15;38(7):110363. doi: 10.1016/j.celrep.2022.110363. PubMed PMID: 35172147; PubMed Central PMCID: PMC8898380.
Semwal MK, Jones NE, Griffith AV. Metabolic Regulation of Thymic Epithelial Cell Function. Front Immunol. 2021;12:636072. doi: 10.3389/fimmu.2021.636072. eCollection 2021. Review. PubMed PMID: 33746975; PubMed Central PMCID: PMC7968369.
Venables T, Griffith AV, DeAraujo A, Petrie HT. Dynamic changes in epithelial cell morphology control thymic organ size during atrophy and regeneration. Nat Commun. 2019 Sep 27;10(1):4402. doi: 10.1038/s41467-019-11879-2. PubMed PMID: 31562306; PubMed Central PMCID: PMC6765001.
Cepeda S, Griffith AV. Thymic stromal cells: Roles in atrophy and age-associated dysfunction of the thymus. Exp Gerontol. 2018 May;105:113-117. doi: 10.1016/j.exger.2017.12.022. Epub 2017 Dec 24. Review. PubMed PMID: 29278750; PubMed Central PMCID: PMC5869099.
Cepeda S, Cantu C, Orozco S, Xiao Y, Brown Z, Semwal MK, Venables T, Anderson MS, GriffithAV. Age-Associated Decline in Thymic B Cell Expression of Aire and Aire-Dependent Self-Antigens. Cell Rep. 2018 Jan 30;22(5):1276-1287. doi: 10.1016/j.celrep.2018.01.015. PubMed PMID: 29386114; PubMed Central PMCID: PMC5813500.
Griffith A.V., Venables T, Shi J, Farr A, Van Remmen H, Szweda L, Fallahi M, Rabinovitch P, Petri H. 2015 Metabolic damage and premature thymus aging caused by stromal catalase deficiency. Cell Reports. Aug 18; 12 (7):1071-1079.
Bryson JL, Griffith A.V., Hughes B, Saito F, Takahama Y, Richie ER, Manley NR. 2013 Cell-Autonomous Defects in Thymic Epithelial Cells Disrupt Endothelial – Perivascular Cell Interactions in the Mouse Thymus. PLoS ONE 8(6), e65196
Griffith A.V., Fallahi M, Venables T, Petrie HT. 2012 Persistent degenerative changes in thymic organ function revealed by an inducible model of organ regrowth. Aging Cell Feb; 11 (1): 169-77.
Griffith A.V., Fallahi M, Nakase H, Gosink M, Young B, Petrie HT. 2009 Spatial mapping of thymic stromal microenvironments reveals unique features influencing T lymphoid differentiation. Immunity Dec 18; 31(6):999-1009.
Griffith A.V., Cardenas K, Carter C, Gordon J, Iberg A, Engleka K, Epstein JA, Manley NR, Richie ER. 2009 Increased thymus- and decreased parathyroid-fated organ domains in Splotch mutant embryos. Dev Biol. Mar 1;327(1):216-27.
Benavides F, Gomez G, Venables-Griffith A, Lambertz I, Flores M, Angel JM, Fuchs-Young R, Richie ER, Conti CJ. 2006 Differential susceptibility to chemically-induced thymic lymphomas in SENCARB and SSIN inbred mice. Mol Carcinog. Jul;45(7):543:8.
Benavides F*, Venables A*, Poetschke Klug H, Glasscock E, Rudensky A, Gomez M, Palenzuela N, Guenet J, Richie E, Conti C. 2001 The CD4 T cell-deficient mouse mutation nackt (nkt) involves a deletion in the cathepsin L (Ctsl) gene. Immunogenetics. Apr;53(3):233-242.*These authors contributed equally to this work.