Mark S. Shapiro, Ph.D.
Professor
Personal Statement:
The research program of the Shapiro lab spans the physiology and modulation of voltage-gated K+ and Ca2+ channels in neurons and non-excitable cells. We have focused on voltage-gated “M-type” (KCNQ) potassium and calcium ion channels, and signaling pathways of G-protein coupled receptors, using patch-clamp electrophysiology of native neurons and heterologous systems, biochemistry, structural biology, confocal and TIRF microscopy, molecular biology and live single-cell and whole-animal imaging. Our major publications have shown the PIP2 sensitivity of both types of channels, the mechanisms and structural determinants of receptor-mediated suppression of IM and ICa, the modulation of KCNQ channels by calmodulin, A-kinase anchoring proteins (AKAPs) and Src kinase, the roles of M channels in airway smooth muscle, and in sensory neurons.
We also seek to systematically explore the role of AKAP79/150 in orchestrating transcriptional and regulatory control of M/KCNQ channels in sympathetic and sensory neurons. We use STORM super-resolution nanoscopy to probe the multi-protein complexes underlying modulation of ion channels. We are also focusing on novel strategies to prevent acquired epilepsies, and the role of M channel regulation in epileptogenesis using brain-slice electrophysiology, single live-cell imaging and transgenic mouse models. Additionally we also are exploring novel and provocative roles of M/KCNQ channels as a neuroprotective mechanism during cerebrovascular ischemic stroke and traumatic brain injury, and chronic pain, to prevent the development of epilepsy and pathological co-morbidities and to develop novel non-opiate approaches to treating chronic pain. We are interested in the ion currents and signaling pathways regulating excitability of dopaminergic neurons in midbrain, so as to understand the pathophysiology of drug addiction, as well as ion channels in lymphocytes critical to lymphomas. Finally, we investigate the mechanisms by which SARS-CoV2 attack the brain, and of the variety of “Long-Covid” (PASC) syndromes affecting millions.
Education
Ph.D., Physiology, Rush University Medical Center, 1991
B.A., Physics, University of Chicago, 1984
Research
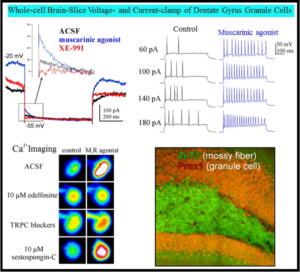 The research program of the Shapiro laboratory centers on the physiology, structure and regulation of voltage-gated K+ and Ca2+, TRP and Cl– ion channels that serve multiple roles in nerve and muscle, as well as their roles as novel therapeutic targets in myriad diseases of the nervous system. The K+ channels are “M-type”, originally called such from their inhibition by stimulation of muscarinic acetylcholine receptors in sympathetic neurons. These K+ channels are widely expressed throughout the peripheral and central nervous system. We study the physiology, modulation and functional role of these channels in heterologous expression systems, dissociated neurons and in brain slice from the hippocampus and other regions. M channels, composed of subunits encoded by the KCNQ (Kv7) family of genes, play fundamental roles in the nervous and cardiovascular systems, inner ear and epithelia. We study regulation of M-channels by a variety of receptors coupled to Gq/11 G proteins, via numerous cytoplasmic 2nd-messingers, including lipids,
The research program of the Shapiro laboratory centers on the physiology, structure and regulation of voltage-gated K+ and Ca2+, TRP and Cl– ion channels that serve multiple roles in nerve and muscle, as well as their roles as novel therapeutic targets in myriad diseases of the nervous system. The K+ channels are “M-type”, originally called such from their inhibition by stimulation of muscarinic acetylcholine receptors in sympathetic neurons. These K+ channels are widely expressed throughout the peripheral and central nervous system. We study the physiology, modulation and functional role of these channels in heterologous expression systems, dissociated neurons and in brain slice from the hippocampus and other regions. M channels, composed of subunits encoded by the KCNQ (Kv7) family of genes, play fundamental roles in the nervous and cardiovascular systems, inner ear and epithelia. We study regulation of M-channels by a variety of receptors coupled to Gq/11 G proteins, via numerous cytoplasmic 2nd-messingers, including lipids, 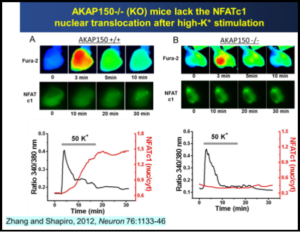 protein kinases and phosphatases, and intracellular Ca2+ ions acting through as calmodulin. Thus, the Shapiro laboratory performs neuroscience research using Biophysical, Molecular, Cellular, Genetic and Behavioral approaches. Our work is very collaborative in nature, involving investigators in other departments at UT Health SA, other institutions in the USA, and many around the world. In the past several years, we have also focused on transcriptional regulation of expression of the channels, both in normal and pathophysiological states, such as during epilepsy, stroke and traumatic brain injury. We are probing the mechanisms of epileptogenesis, and how to prevent its development, focusing on the temporal lobe, particularly circuits in the hippocampus. We also study the regulation of N- and P/Q- and L-type voltage-gated Ca2+ channels, which drive exocytosis, release of neurotransmitter at nerve terminals, and transcriptional regulation, and whose modulation is a prime mechanism of synaptic plasticity. Our novel imaging and electrophysiology studies have broadened to include TRP and ASIC cation channels, Ano Ca2+-activated Cl– channels, and others.
protein kinases and phosphatases, and intracellular Ca2+ ions acting through as calmodulin. Thus, the Shapiro laboratory performs neuroscience research using Biophysical, Molecular, Cellular, Genetic and Behavioral approaches. Our work is very collaborative in nature, involving investigators in other departments at UT Health SA, other institutions in the USA, and many around the world. In the past several years, we have also focused on transcriptional regulation of expression of the channels, both in normal and pathophysiological states, such as during epilepsy, stroke and traumatic brain injury. We are probing the mechanisms of epileptogenesis, and how to prevent its development, focusing on the temporal lobe, particularly circuits in the hippocampus. We also study the regulation of N- and P/Q- and L-type voltage-gated Ca2+ channels, which drive exocytosis, release of neurotransmitter at nerve terminals, and transcriptional regulation, and whose modulation is a prime mechanism of synaptic plasticity. Our novel imaging and electrophysiology studies have broadened to include TRP and ASIC cation channels, Ano Ca2+-activated Cl– channels, and others. 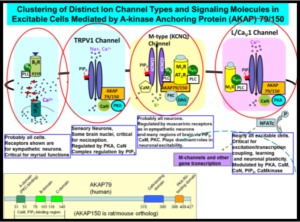 Several neurophysiological approaches are used, including preparations of primary neurons from the peripheral and central nervous systems and heterologous systems in which channels, receptors, and signaling molecules are transfected into mammalian tissue-culture cells. Our work is greatly enhanced by the ability to manipulate expression of exogenous genes, or to “knock down” endogenous ones, using transgenic mice. The experimental techniques include patch-clamp electrophysiology, cutting-edge molecular biology, advanced imaging techniques such as total internal reflection fluorescence (TIRF) microscopy, Förster resonance energy transfer (FRET), confocal and “super-resolution” microscopy, molecular and cellular modeling, biophysical chemical analysis of channel complexes and structural biology.
Several neurophysiological approaches are used, including preparations of primary neurons from the peripheral and central nervous systems and heterologous systems in which channels, receptors, and signaling molecules are transfected into mammalian tissue-culture cells. Our work is greatly enhanced by the ability to manipulate expression of exogenous genes, or to “knock down” endogenous ones, using transgenic mice. The experimental techniques include patch-clamp electrophysiology, cutting-edge molecular biology, advanced imaging techniques such as total internal reflection fluorescence (TIRF) microscopy, Förster resonance energy transfer (FRET), confocal and “super-resolution” microscopy, molecular and cellular modeling, biophysical chemical analysis of channel complexes and structural biology. 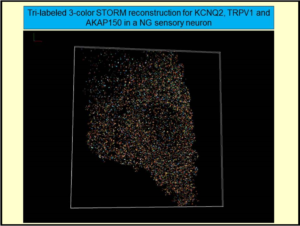 Our recent discoveries include findings that ion channels, receptors and signaling proteins are clustered into discrete “nanodomains” that orchestrate directed signals in ganglia and brain, nanodomains which we have visualized using visible light at 5 nm resolution via STORM “nanoscopy.” Such nanodomain complexes of proteins direct neuronal excitability in distinct and purposeful ways, guide transcriptional expression, and underlie plasticity, cognition and circuit development in brain. We have carefully mapped out the multiple domains of KCNQ ion channels involved in interactions with phosphatidylinositol 4,5-bisphosphate (PIP2), a lipid signaling molecule of critical importance. Moving into structural biological techniques of X-ray crystallography and NMR spectroscopy and biophysical chemical analysis, we have obtained the structure of Ca2+/calmodulin bound to a KCNQ channel, yielding insights into how diverse intracellular signals interact on a molecular level. Finally, our multiple translational neuroscience projects have unveiled novel therapeutic interventions that seem likely to prevent epilepsy, cognitive dysfunction and mood disorders after traumatic brain injury and stroke, and novel approaches to prevent epilepsy from developing, or new approaches to counter-acting this disorder in people.
Our recent discoveries include findings that ion channels, receptors and signaling proteins are clustered into discrete “nanodomains” that orchestrate directed signals in ganglia and brain, nanodomains which we have visualized using visible light at 5 nm resolution via STORM “nanoscopy.” Such nanodomain complexes of proteins direct neuronal excitability in distinct and purposeful ways, guide transcriptional expression, and underlie plasticity, cognition and circuit development in brain. We have carefully mapped out the multiple domains of KCNQ ion channels involved in interactions with phosphatidylinositol 4,5-bisphosphate (PIP2), a lipid signaling molecule of critical importance. Moving into structural biological techniques of X-ray crystallography and NMR spectroscopy and biophysical chemical analysis, we have obtained the structure of Ca2+/calmodulin bound to a KCNQ channel, yielding insights into how diverse intracellular signals interact on a molecular level. Finally, our multiple translational neuroscience projects have unveiled novel therapeutic interventions that seem likely to prevent epilepsy, cognitive dysfunction and mood disorders after traumatic brain injury and stroke, and novel approaches to prevent epilepsy from developing, or new approaches to counter-acting this disorder in people.
Awards & Accomplishments
President and Officer, Society of General Physiology, 2013-15 (Elect), 2015-2017 (President), 2018-2020 (ex-President). President’s Council Faculty Scholar Award, UT Health SA (UTHSCSA), 2013-2015. Journal of Neuroscience, Associate Editor, 2012-present. National Institute of Health, Study Section (IRG) Standing Panel Member: 2007-2010, 2017-present.
Affiliations
Biophysical Society, 1987-present.
Society for Neuroscience 1991-present.
Society of General Physiology, 2002-present.
National Neurotrauma Society, 2016-present.
Sam and Ann Barshop Institute for Longevity and Aging Studies, 2023-present.
Center for Mitochondrial Medicine, 2023-present.
Lab Members
Shapiro Lab
 |
 | Fabio Borges Vigil, Ph.D. Instructor/Research |
| Angel David Nunez Correa Research Assistant |
|
 | Elliot Morgan Student Associate |
News
2023
- Invited Symposium Speaker, 2nd International Symposium on Kv7 K+ ion channels, Naples, Italy. Seminar, University of Leeds, U.K.
2024
- Invited Panelist/Speaker, Winter Brain Research Meeting, Breckenridge, CO
- Invited Keynote Lecture, Mississippi Neurotrauma Society, Jackson, MI.
- Invited Seminar Speaker, Center for Neuroscience and Cell Biology, University of Coimbra, Coimbra, Portugal
DoD CDMRP Phase 1, (PI: F.A. Vigil Ph.D, co-I, M.S. Shapiro),. 5/1/22-4/30/24. “Acute pharmacological augmentation of Kv7 K+ ion channels prevents post-traumatic epilepsy and chronic traumatic encephalopathy.” Direct Costs: $300,000.
DoD, CDMRP (PI: C.M. Carver), “Neuron-selective neuromodulation and TRPC ion channel targeting for prevention of trauma-induced temporal lobe epilepsy,” 10/1/19-7/318/21, Direct/Indirect/Total Costs: $296,141/$149,203/$445,344.
Publications
|
Vigil F.A., Belchior H., Bugay V.A., Cortes D, Chun S.E., Holstein, D.M., Cavazos J.E., Lechleiter J.D. Brenner R and M.S. Shapiro (2023). Acute pharmacological augmentation of “M-type” (Kv7, KCNQ) K+ currents as a treatment for the long-term effects of repetitive blast traumatic brain injuries. Neurotherapeutics. 20(3):853-869.
|
|
Dixon G., Shapiro M.S., Potter S., Ni, A., Xu N., Santa Maria D., and C.I. Brady. Platelet Rich Plasma Induced Remodeling of the Abductor Pollicis Longus Tendon, a Treatment for De Quervain’s Tenosynovitis. Current Sports Medicine Reports (Submitted)
|
|
Dixon G., Potter, S., Shapiro, M.S., Santa Maria, E., Schmidt, D. and D. Santa Maria (2023). Profound Healing of a Quadriceps Tendon Tear Following Intratendinous Minimally Invasive Platelet-Enriched Plasma Injection. Current Sports Medicine Reports. 22(5):p 164-167, May 2023.
|
|
Carver, C.M., DeWitt, H.R, Stoja, A.P. and M.S. Shapiro. (2021). Blockade of TRPC channels limits cholinergic-driven hyperexcitability and seizure susceptibility after traumatic brain injury. Frontiers in Neuroscience15:681144.
|
|
Bozdemir, E., Vigil, F.A., Bugay, V., Espinoza, L., Chun, S.H., Hobbs, M., Khoury, S., Holstein, D., Sanchez, I., Cavazos, J., Brenner, R., Shapiro, M.S. and Lechleiter, J.D. (2021). Novel agonists of Adenosine A3 receptors prevent brain damage after traumatic brain injury. Neurotherapeutics Oct 4, 2021.
|
|
Vigil, F.A., Carver, C.M. and M.S. Shapiro. (2020). Pharmacological manipulation of Kv7 channels as a new therapeutic tool for multiple brain disorders. Frontiers in Physiology 11:688.
|
|
Shah, S., Carver, C., Shapiro, M.S., Gamper, N. (2020). Nanodomain Ca2+signals couple activation of TRPV1 and ANO1 sensory neuron on channels. Science Signaling 13:
|
|
Carver, C.M., Hastings, S.D., Cook, M.E., and M.S. Shapiro. (2020). Functional responses of the hippocampus to hyperexcitability depend on directed, neuron-specific KCNQ2 K+ channel plasticity. Hippocampus30(5):435-455.
|
|
Bugay, V., Bozdemir, E., Vigil, F. A., Holstein, D. M., Elliot, E., Sprague, C., Rule, G., Cavazos, J., Lund, B., Shapiro, M. S., Lechleiter, J. D. and R. Brenner. (2020). A mouse model of repetitive blast trauma head injury reveals a high incidence of acute generalized seizures. J. Neurotrauma 37:248–261.
|
| Vigil, F.A., Bozdemir, E., Veraza, R.J., Bugay, V., Holstein, D.M., Hobbs, M, Sanchez, I, Cavazos, J, Brenner, R, Lechleiter, J.D. and M.S. Shapiro. (2019). Prevention of brain damage after traumatic brain injury by pharmacological enhancement of KCNQ (Kv7, ‘‘M-type’’) K+ currents in neurons J. Cerebral Blood Flow & Metabolism. Published on-line July 4th, 2019. |
| Archer, C.R., Enslow, B., Bhattacharya, A., Taylor, A. and M.S. Shapiro. (2019). A mutually-induced conformational fit underlies Ca2+-directed interactions between calmodulin and the proximal carboxy terminus of KCNQ4 K+ channels. 294:6094-6112. |
| Carver C.M. and M.S. Shapiro (2019). Divergent region-specific regulation of neuronal excitability by muscarinic Gq-coupled receptors via M-type K+ and TRPC cation channels in the hippocampus J. Neurosci. 39:1566-1587. |
|
Choveau, F., Zhang, J., De la Rosa, V., Hernandez, C., C. and M. S. Shapiro. (2018). Phosphatidylinositol 4,5-bisphosphate (PIP2) regulates KCNQ3 K+ channels through multiple sites of action J. Biol. Chem. 293(50):19411-28.
|
| Carver, C.C. and M.S. Shapiro (2018). Divergent region-specific regulation of neuronal excitability by muscarinic Gq-coupled receptors via M-type K+ channels and TRPC cation channels in the hippocampus (Submitted to Neuron). |
| Zhang, J., Carver, C.C., Choveau F., and M.S. Shapiro. (2016). Clustering and functional coupling of diverse ion channels and signaling proteins revealed by super-resolution STORM microscopy in neurons. Neuron. Oct 19;92(2):461-478. |

