Research Highlights
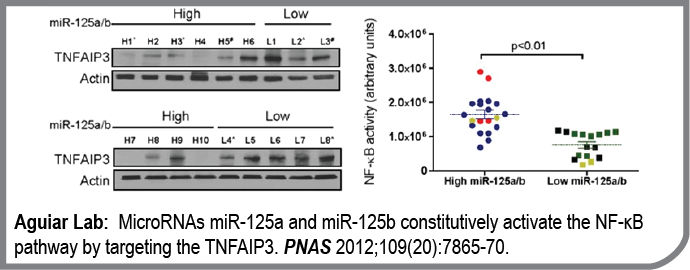
In this work, we established the contribution of microRNA dysfunction to the constitutive activation of the NF-kB pathway, the hallmark of the most aggressive subtypes of B-cell lymphomas.
Building on our previous genome-wide investigation of microRNA copy number/expression in lymphomas, and integrating these data with microRNA target prediction models, we identified miR-125a and miR-125b as direct inhibitors of the NF-kB negative regulator TNFAIP3. Using multiple genetic models of gain and loss of function, we confirmed that miR-125a/b, by suppressing TNFAIP3, constitutively activates the NF-kB pathway, enhance the fitness of lymphoma cells, and render them resistance to apoptosis. Importantly, we showed that a sizable fraction of primary human lymphomas overexpress these miRNAs and, consequently, display higher NF-kB activity. Novel therapeutic approaches are greatly needed for lymphomas with aberrant NF-kB function, and our work provides the rational for using anti-miR-125a/b strategies in this clinical setting.
- MicroRNAs miR-125a and miR-125b constitutively activate the NF-κB pathway by targeting the tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20).
Kim SW, Ramasamy K, Bouamar H, Lin AP, Jiang D, Aguiar RC
Proc Natl Acad Sci U S A: 2012-05-15; 109(20); 7865-70 Epub: 2012-05-01.
PMID: 22550173
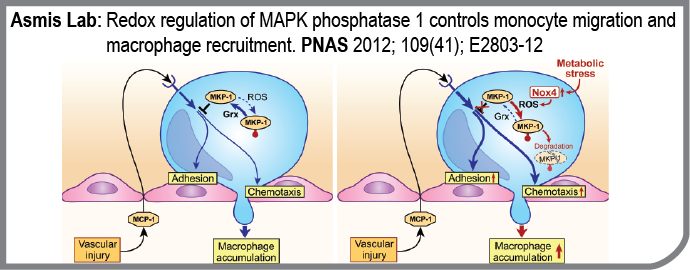
Metabolic disorders such as obesity and diabetes are associated with a state of chronic, low-grade inflammation, which appears to contribute to the development of micro- and macrovascular complications such as atherosclerosis, nephropathy, and retinopathy. The cellular and molecular mechanisms involved in chronic inflammation associated with metabolic disorders are not yet fully understood, but the recruitment of blood monocytes to sites of vascular injury appears to play a central and rate-limiting role in all these complications. The Asmis Lab recently reported that metabolic disorders “prime” monocytes for enhanced recruitment into vascular lesions by increasing monocytes’ responsiveness to chemoattractants. In this paper they provide molecular details of this novel proatherogenic mechanism and identified mitogen activated protein kinase phosphatase 1 (MKP-1) as a as a central redox-sensitive regulator of monocyte adhesion and migration.
- Redox regulation of MAPK phosphatase 1 controls monocyte migration and macrophage recruitment.
Kim HS, Ullevig SL, Zamora D, Lee CF, Asmis R.
Proc Natl Acad Sci U S A. 2012 Oct 9;109(41):E2803-12. doi: 10.1073/pnas.1212596109. Epub 2012 Sep 18.; PubMed Central PMCID: PMC3478659.
PMID: 22991462
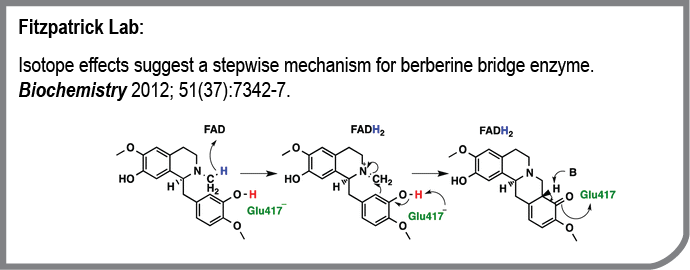
The Fitzpatrick lab is interested in the catalytic and regulatory mechanisms of enzymes. We selected the flavoprotein berberine bridge enzyme for study because it catalyzes an unusual reaction in the biosynthesis of plant alkaloids that are of interest as drugs. In this publication we addressed the timing of two steps in the catalytic reaction: the oxidation of the substrate by the enzyme-bound flavin and the formation of the new carbon-nitrogen bond. We replaced reactive hydrogens with deuterium to slow each of these steps and then measured the change in the rate constant for formation of the product on the enzyme in a single turnover. We also mutated the active site base to determine its role. The results allow us to propose a detailed reaction mechanism for the enzyme.
- Isotope effects suggest a stepwise mechanism for berberine bridge enzyme.
Gaweska HM, Roberts KM, Fitzpatrick PF
Biochemistry: 2012-09-18; 51(37); 7342-7 Epub: 2012-09-06.
PMID: 22931234
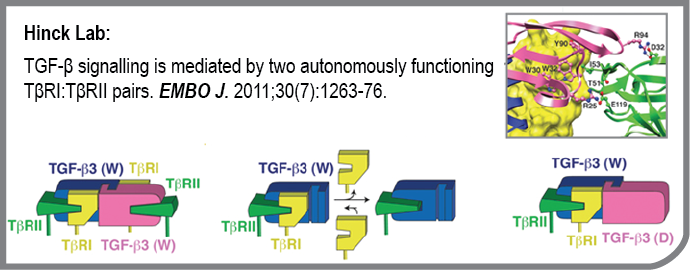
One of the defining characteristics of cancer cells is their ability to rapidly proliferate, evade the host immune system, invade the surrounding tissue, and metastasize to distant organs and tissues. Cancer cells often attain these aggressive characteristics by altering the function of normal cellular proteins to favor these activities. Cell signaling pathways, such as that of transforming growth factor-beta or TGF-beta, are often dysregulated to favor these aggressive characteristic during carcinogenesis. One of the emerging strategies in cancer treatment, especially for aggressive cancers such as metastatic breast and prostate cancer, is to therefore block proteins such as TGF-beta so the cancer cells can no longer misuse them. Our laboratory, which has studied TGF-beta structure and function for more nearly 20 years is working together to block TGF-beta by preventing it from interacting with its receptors, the critical first step in the TGF-beta signaling pathway. Our efforts in this regard have been made possible by the determination of the atomic level structure of TGF-beta bound to its receptors, which has shown how the different receptors are arranged relative to one another when they bind TGF-beta (Groppe, et al Mol. Cell, 29, 157-168 (2008)).
One of the important questions not resolved by the structure of the TGF-beta receptor complex is whether the two type I and two type II receptors (known as TβRI and TβRII, respectively) that comprise the TGF-beta receptor complex are required for signaling, or whether a single type I and type II receptor might suffice. This question was addressed by generating a heterodimeric form of TGF-beta comprised of one wild type monomer and one monomer bearing substitutions to block receptor binding. The receptor binding properties of the purified heterodimer were then studied using highly quantitative surface plasmon resonance (SPR) measurements. The SPR results showed that heterodimeric TGF-beta bound TβRII and recruited TβRI with affinities indistinguishable from the wild type homodimer, but with one half the stoichiometry. To complete this study, an extensive analysis of the biological activity of the heterodimer was performed, as well as an analysis of the receptor complexes assembled on the cell surface using single-molecule fluorescence. The results very clearly showed that the two TβRI:TβRII heterodimers function independently of one another. These results show that inhibition of TGF-beta signaling requires simultaneously blocking of the type I and type II receptor binding sites on both sides of the TGF-beta homodimer. This important insight, together with previously determined structure of the TGF-beta receptor complex, are now being used by the Hinck laboratory to develop novel receptor fusions that bind TGF-beta and attenuate its tumor-promoting activity in advanced breast and prostate cancer.
- TGF-β signalling is mediated by two autonomously functioning TβRI:TβRII pairs.
Huang T, David L, Mendoza V, Yang Y, Villarreal M, De K, Sun L, Fang X, López-Casillas F, Wrana JL, Hinck AP
EMBO J: 2011-04-06; 30(7); 1263-76 Epub: 2011-03-18.
PMID: 21423151
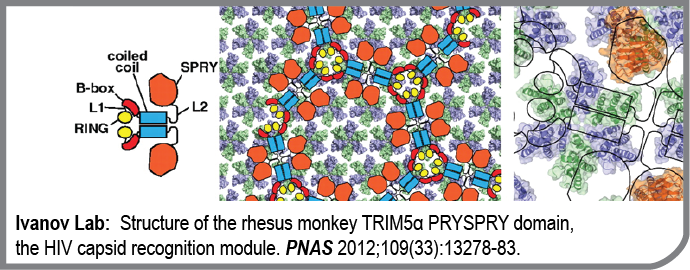
TRIM5alpha proteins bind retroviral capsids after cell entry and restrict retroviral infection by blocking reverse transcription and/or integration of the viral genetic material. This novel mechanism of cellular immunity against retroviruses appears to determine the species tropism of the primate immunodeficiency viruses active today. Species-specific differences in TRIM5alpha activity arise from differences in TRIM5alpha affinity for the capsid. For example, human TRIM5alpha binds HIV capsid weakly and does not restrict HIV. Remarkably, deletion of a single amino acid in huTRIM5alpha restores its affinity for the HIV capsid and HIV restriction. Capsid recognition is mediated by the B30.2 domain of TRIM5alpha, but the recognition mechanism is still not known. This publication from the Ivanov Lab provides an important piece of the recognition puzzle. The group succeeded in determining the elusive structure of the SPRY domain from rhesus monkey using an elegant combination of NMR spectroscopy and X-ray crystallography. These results open the way for modeling of this host-pathogen interface that may hold the key to the understanding of why humans are susceptible to HIV/AIDS.
- Structure of the rhesus monkey TRIM5α PRYSPRY domain, the HIV capsid recognition module.
Biris N, Yang Y, Taylor AB, Tomashevski A, Guo M, Hart PJ, Diaz-Griffero F, Ivanov DN
Proc Natl Acad Sci U S A: 2012-08-14; 109(33); 13278-83 Epub: 2012-07-30.
PMID: 22847415
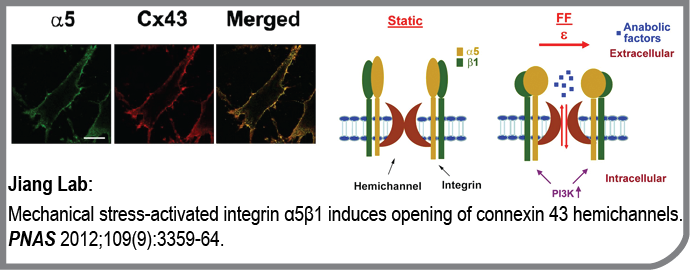

This publication from the Jiang lab was highlighted by the editorial board of the Science magazine as an “editors choice” publication in cell biology. Here is what they wrote:
A Forced Opening
Mechanical forces on bone stimulate intracellular signaling pathways in osteocytes that promote remodeling. Signaling molecules that drive such remodeling are likely transmitted between cells through the connexin 43 (Cx43) hemichannel (HC). Integrins are focal adhesion proteins that provide support to the cell by connecting the cytoskeleton to extracellular matrix (ECM) components like fi bronectin. Integrins have also been proposed to be mechanosensors in bone cells. Batra et al. now show that the cytoplasmic domain of the α subunit of integrin α5β1 interacts with the cytoplasmic domain of Cx43, that the interaction is strengthened by fl uid fl ow, and that this interaction is required for HC opening. The opening required osteocyte attachment to the ECM but was independent of integrin α5β1 binding to its ligand, fi bronectin. Direct forces applied to integrin α5β1 by magnetic beads induced the opening of the HC, and such mechanical stress–induced opening required phosphatidylinositol 3-kinase (PI3K), which has been implicated in fl uid-fl ow–induced activation of integrins in epithelial cells. It is likely that PI3K is responsive to fl uid fl ow and transmits this to integrins, leading to opening of the HC and bone remodeling.
- Mechanical stress-activated integrin α5β1 induces opening of connexin 43 hemichannels.
Batra N, Burra S, Siller-Jackson AJ, Gu S, Xia X, Weber GF, DeSimone D, Bonewald LF, Lafer EM, Sprague E, Schwartz MA, Jiang JX
Proc Natl Acad Sci U S A: 2012-02-28; 109(9); 3359-64 Epub: 2012-02-13.
PMID: 22331870
NADPH-cytochrome P450 reductase (POR) is the sole source of electrons for 50 microsomal cytochromes P450 in human liver. The substrates for these are both endobiotics and xenobiotics and vary from therapeutic drugs to aryl hydrocarbons to fatty acids to steroids. The total knockout of POR in mice is embryonically lethal in mice and numerous human deficiencies caused by missense mutations have been described. Xia et al. (PNAS, 2011) revealed the crystal structure of NADP+-bound wild-type POR and two FAD-binding mutants that revealed the structural aberrations resulting in their dysfunction. Previous studies in Dr. Masters’ lab have shown that the enzymatic activities of these POR variants can be rescued by the addition of the flavin cofactor, FAD, and the structures reveal the basis for the reversal of these defects.
- Structural basis for human NADPH-cytochrome P450 oxidoreductase deficiency.
Xia C, Panda SP, Marohnic CC, Martásek P, Masters BS, Kim JJ
Proc Natl Acad Sci U S A: 2011-08-16; 108(33); 13486-91 Epub: 2011-08-01.
PMID: 21808038
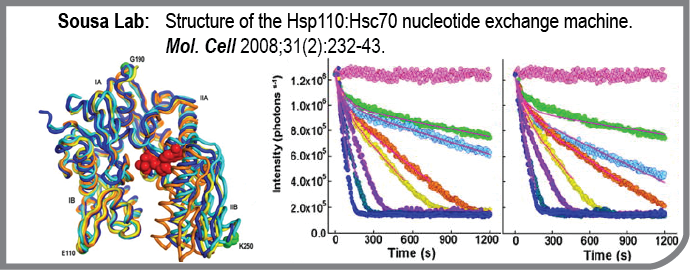
This study from the Sousa lab reports the structure and functional characterization of the Hsp70:Hsp110 nucleotide exchange complex. Hsp70 mediates numerous protein processing and quality control reactions in all organisms, from bacteria to humans. Hsp70 binding and release of the proteins it works on is controlled by nucleotide binding, and nucleotide binding and release from Hsp70 is, in turn, regulated by Hsp110. Studies in the Sousa lab have revealed how Hsp110 induces a conformational change in Hsp70 that results in release of bound nucleotide. The Hsp70:Hsp110 complex is an essential component of the protein repair machinery whose dysfunction can lead to neurodegenerative disease.
- Structure of the Hsp110:Hsc70 nucleotide exchange machine.
Schuermann JP, Jiang J, Cuellar J, Llorca O, Wang L, Gimenez LE, Jin S, Taylor AB, Demeler B, Morano KA, Hart PJ, Valpuesta JM, Lafer EM, Sousa R
Mol Cell: 2008-07-25; 31(2); 232-43 Epub: 2008-06-12.
PMID: 18550409
